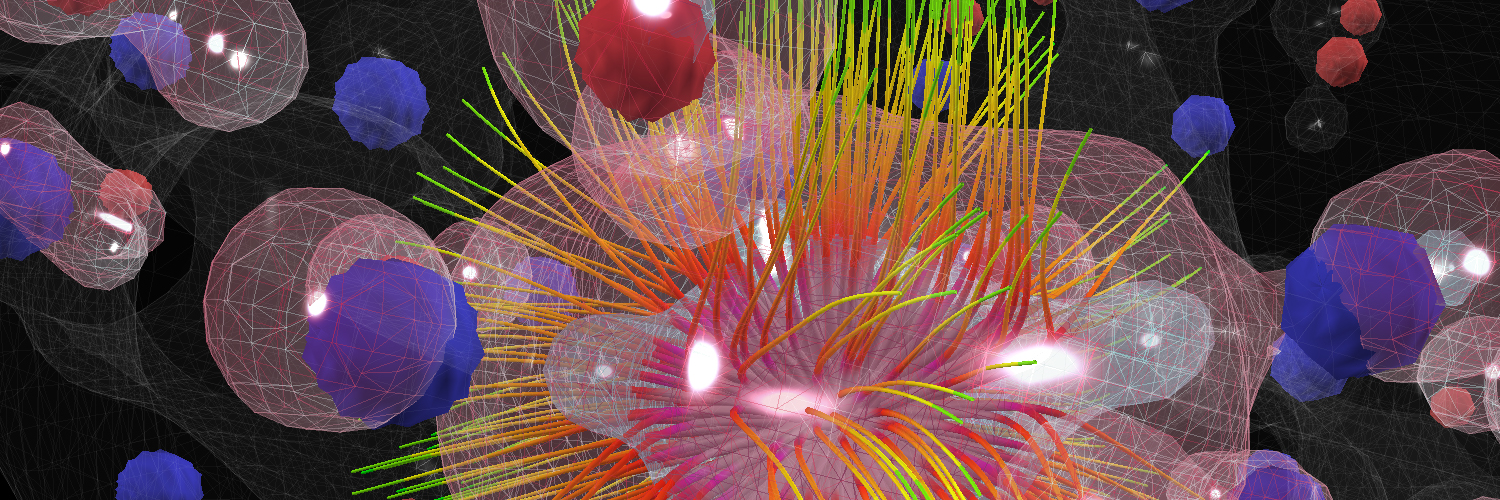
Multi-Center Hydrogen Bond
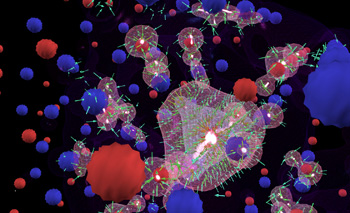
In this multi-center hydrogen bond demo, the source of conductivity is zinc. Hydrogen replaces oxygen and forms a highly unusual multi-center bond. Simulations will allow for calculations at a higher level of complexity, leading to the investigation of how bonding strength changes as hydrogen is gradually drawn out of a hydride compound. This is a technique for using hydrogen as an alternative energy source, functioning as it would in a real world hydrogen car. The research is focusing on substances that hold hydrogen like a sponge, with the hydrogen atoms bonded weakly to the crystal structure of the host material so that they can be released with a small amount of heat. Visualizations and interactive simulations are leading to new discoveries on how these materials bond and can be released. One flies through the 2000 atom lattice navigating by the sonification of the atomic emission spectra of oxygen and zinc. The unique hydrogen bond has its own "musical voice": all sonic information comes from precise mathematical calculations transposing the atomic emission spectra into the audio domain.
Key faculty, postdoctoral, and graduate student researchers associated with the project: Professor Chris Van de Walle, Dr. Anderson Janotti, Professor JoAnn Kuchera-Morin, Dr. Lance Putnam, Dr. Basak Alper.